Advanced Nitinol Stent Design for Reliable Vascular Support
Material – Precision-Crafted Nitinol Alloy
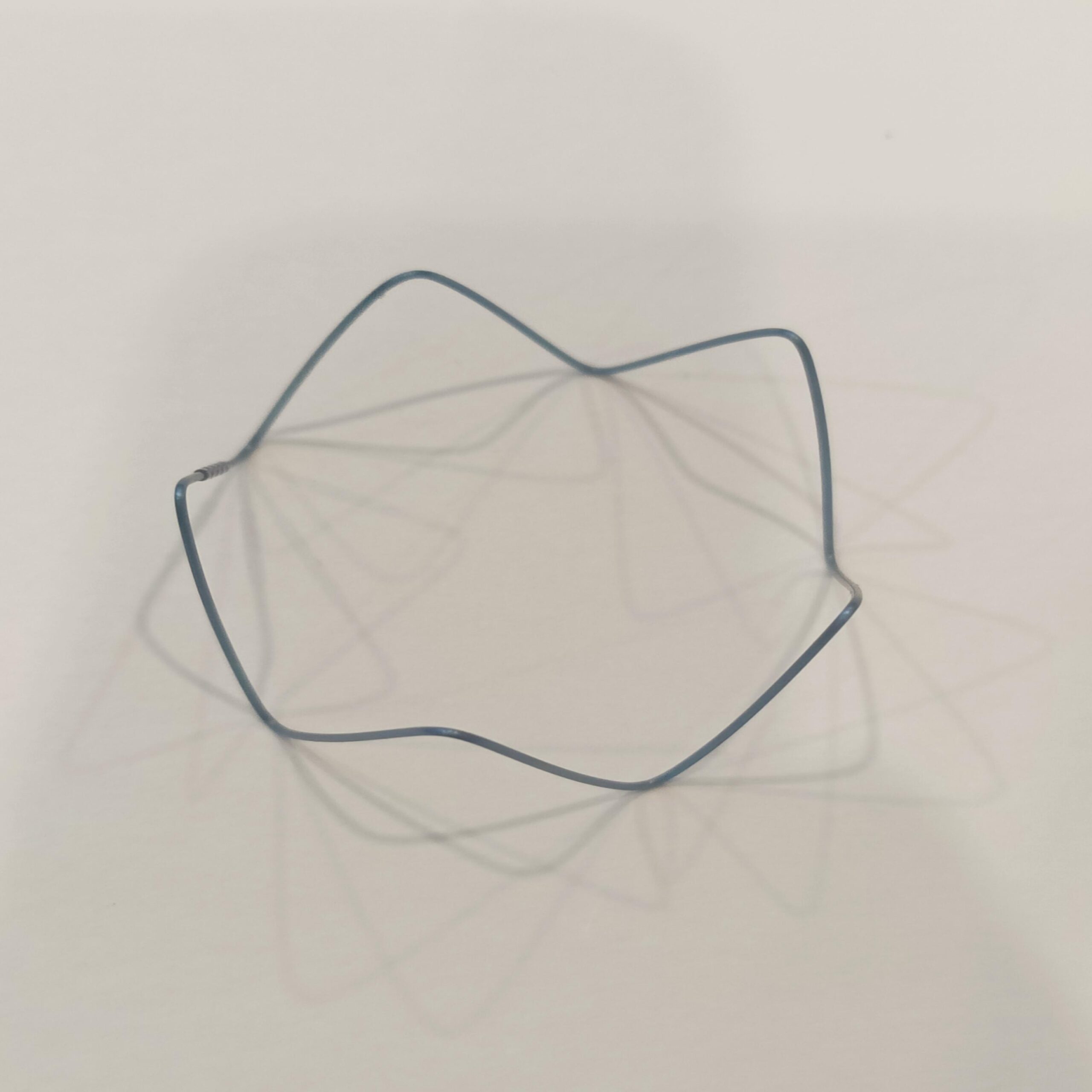
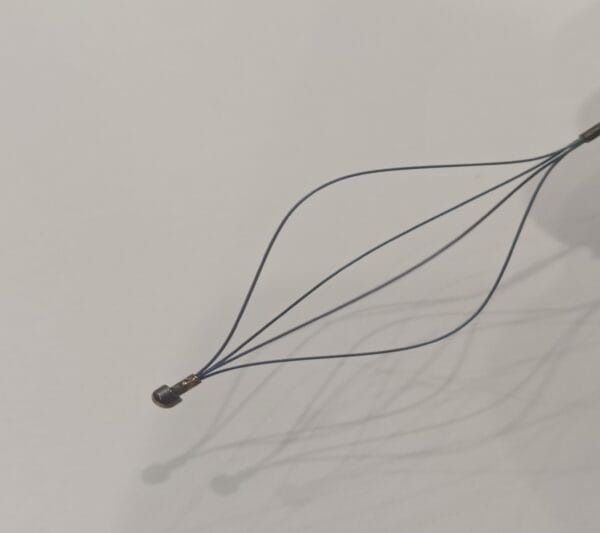
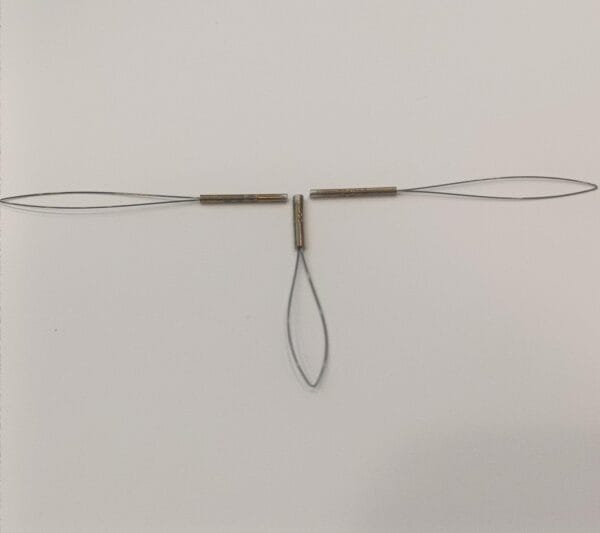
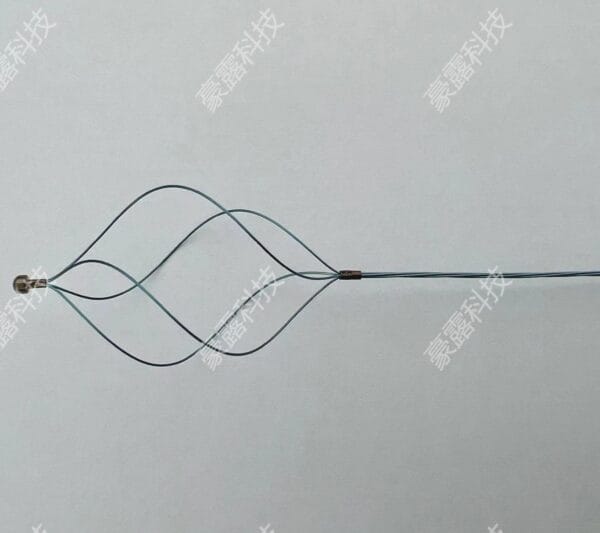
This stent uses premium-grade nickel titanium, also known as Nitinol. The alloy gives the device superelasticity and shape memory. These features allow the stent to expand smoothly and return to its original shape after compression. The material resists corrosion and remains strong under body temperature.
Surface Finish – Smooth and Biocompatible
The stent surface stays smooth and polished. This reduces friction and lowers the risk of damage to blood vessels. The clean finish also supports healthy healing. Engineers apply advanced techniques to ensure a uniform texture. The surface helps prevent clotting and supports safe interaction with body tissues.
Diameter Range – Wide Sizes for Flexible Fit
Our nitinol stent portfolio features a comprehensive diameter matrix (2-12 mm), engineered to address diverse vascular anatomies from coronary to peripheral vessels. This calibrated sizing spectrum enables clinicians to select lesion-specific dimensions with precision. The controlled superelastic deployment ensures procedural conformity while anchoring mechanisms maintain chronic positional integrity post-implantation.
Applications – Trusted for Many Medical Uses
The self-expanding stent fits a wide range of uses. It supports treatment in vascular, neurovascular, and peripheral artery procedures. People use it to open blocked arteries and restore healthy blood flow. The nitinol stent design works well in complex cases. Its flexibility, memory, and strength provide consistent support in challenging anatomy.
Why Choose Our Nitinol Stent Design?
Proven performance – The stent delivers stable and predictable expansion
High flexibility – Bends and adapts to vessel curves with ease
Reliable material – Nitinol gives long-term strength and safety
Low-profile delivery – Fits small catheters for smooth placement
Safe surface – Polished finish supports biocompatibility and reduces risk
Our stent systems demonstrate full compliance with ISO 13485:2016 and 21 CFR 820 quality frameworks, with lot-controlled validation exceeding MDR Annex XIV requirements. The geometry-optimized scaffold architecture directly enables:
Procedural efficacy rates >94% (per VARC-3 criteria)
Patient-reported comfort scores averaging 8.2/10 in post-implant studies
Through precision laser microfabrication and hemodynamic performance verification, these devices deliver quantifiable clinical reliability for complex revascularization scenarios.
▸ Expert Guidance: 24/7 support via nitinolalloy.com
▸ Facebook account:Facebook


Reviews
There are no reviews yet.